Search
- Page Path
- HOME > Search
Songwon Lecture 2022
- Hypothalamus and pituitary gland
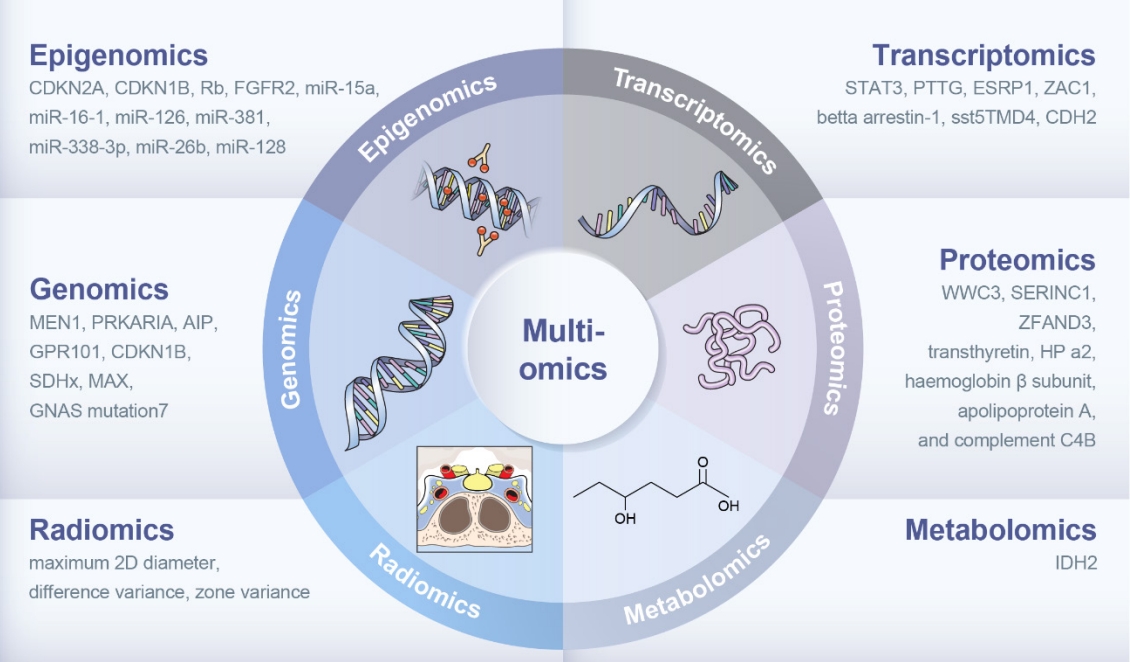
- Multiomics Approach to Acromegaly: Unveiling Translational Insights for Precision Medicine
- Kyungwon Kim, Cheol Ryong Ku, Eun Jig Lee
- Endocrinol Metab. 2023;38(5):463-471. Published online October 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1820
- 1,741 View
- 122 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The clinical characteristics and prognoses of acromegaly vary among patients. Assessment of current and novel predictors can lead to multilevel categorization of patients, allowing integration into new clinical guidelines and a reduction in the increased morbidity and mortality associated with acromegaly. Despite advances in the diagnosis and treatment of acromegaly, its pathophysiology remains unclear. Recent advancements in multiomics technologies, including genomics, transcriptomics, proteomics, metabolomics, and radiomics, have offered new opportunities to unravel the complex pathophysiology of acromegaly. This review comprehensively explores the emerging role of multiomics approaches in elucidating the molecular landscape of acromegaly. We discuss the potential implications of multiomics data integration in the development of novel diagnostic tools, identification of therapeutic targets, and the prospects of precision medicine in acromegaly management. By integrating diverse omics datasets, these approaches can provide valuable insights into disease mechanisms, facilitate the identification of diagnostic biomarkers, and identify potential therapeutic targets for precision medicine in the management of acromegaly.
-
Citations
Citations to this article as recorded by- “Micromegaly”: Acromegaly with apparently normal GH, an entity on its own?
Lucio Vilar, Luciana Ansaneli Naves, Manoel Ricardo Alves Martins, Antônio Ribeiro-Oliveira Jr
Best Practice & Research Clinical Endocrinology & Metabolism.2024; : 101878. CrossRef
- “Micromegaly”: Acromegaly with apparently normal GH, an entity on its own?

Original Article
- Clinical Study
- Associations of GNAS Mutations with Surgical Outcomes in Patients with Growth Hormone-Secreting Pituitary Adenoma
- Hyein Jung, Kyungwon Kim, Daham Kim, Ju Hyung Moon, Eui Hyun Kim, Se Hoon Kim, Cheol Ryong Ku, Eun Jig Lee
- Endocrinol Metab. 2021;36(2):342-350. Published online March 23, 2021
- DOI: https://doi.org/10.3803/EnM.2020.875

- 4,370 View
- 144 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The guanine nucleotide-binding protein, alpha stimulating (GNAS) gene has been associated with growth hormone (GH)-secreting pituitary adenoma. We investigated the prevalence of GNAS mutations in Korean patients with acromegaly and assessed whether mutation status correlated with biochemical or clinical characteristics.
Methods
We studied 126 patients with acromegaly who underwent surgery between 2005 and 2014 at Severance Hospital. We performed GNAS gene analysis and evaluated age, sex, hormone levels, postoperative biochemical remission, and immunohistochemical staining results of the tumor.
Results
GNAS mutations were present in 75 patients (59.5%). Patients with and without GNAS mutations showed similar age distribution and Knosp classification. The proportion of female patients was 76.5% and 48.0% in the GNAS-negative and GNAS-mutation groups, respectively (P=0.006). In immunohistochemical staining, the GNAS-mutation group showed higher GH expression in pituitary tumor tissues than the mutation-negative group (98.7% vs. 92.2%, P=0.015). Patients with GNAS mutations had higher preoperative insulin-like growth factor-1 levels (791.3 ng/mL vs. 697.0 ng/mL, P=0.045) and lower immediate postoperative basal (0.9 ng/mL vs. 1.0 ng/mL, P=0.191) and nadir GH levels (0.3 ng/mL vs. 0.6 ng/mL, P=0.012) in oral glucose tolerance tests. Finally, the GNAS-mutation group showed significantly higher surgical remission rates than the mutation-negative group, both at 1 week and 6 months after surgical resection (70.7% vs. 54.9%, P=0.011; 85.3% vs. 82.4%, P=0.007, respectively).
Conclusion
GNAS mutations in GH-secreting pituitary tumors are associated with higher preoperative insulin-like growth factor-1 levels and surgical remission rates and lower immediate postoperative nadir GH levels. Thus, GNAS mutation status can predict surgical responsiveness in patients with acromegaly. -
Citations
Citations to this article as recorded by- Genetic diagnosis in acromegaly and gigantism: From research to clinical practice
Claudia Ramírez-Rentería, Laura C. Hernández-Ramírez
Best Practice & Research Clinical Endocrinology & Metabolism.2024; : 101892. CrossRef - CD8/PD-L1 immunohistochemical reactivity and gene alterations in cutaneous squamous cell carcinoma
Haruto Nishida, Yoshihiko Kondo, Takahiro Kusaba, Kazuhiro Kawamura, Yuzo Oyama, Tsutomu Daa, Avaniyapuram Kannan Murugan
PLOS ONE.2023; 18(2): e0281647. CrossRef - Dynamic monitoring of circulating tumor DNA to analyze genetic characteristics and resistance profile of lorlatinib in ALK positive previously treated NSCLC
Xiya Ma, Kun Zhang, Jing Xu, Hongjun Gao, Shaoxing Yang, Haifeng Qin, Hong Wang, Fang Gao, Xiaoqing Liu
Thoracic Cancer.2023; 14(20): 1980. CrossRef - Multiomics Approach to Acromegaly: Unveiling Translational Insights for Precision Medicine
Kyungwon Kim, Cheol Ryong Ku, Eun Jig Lee
Endocrinology and Metabolism.2023; 38(5): 463. CrossRef - Hotspots of Somatic Genetic Variation in Pituitary Neuroendocrine Tumors
Mariana Torres-Morán, Alexa L. Franco-Álvarez, Rosa G. Rebollar-Vega, Laura C. Hernández-Ramírez
Cancers.2023; 15(23): 5685. CrossRef
- Genetic diagnosis in acromegaly and gigantism: From research to clinical practice


 KES
KES

 First
First Prev
Prev



